Hepatitis E
Hepatitis E is a waterborne disease caused by the Hepatitis E virus (HEV). Hepatitis E is mainly found in areas with poor sanitation and is typically caused by ingesting fecal matter. This disease is uncommon in the U.S.. However, cases of Hepatitis E have been reported in the Middle East, Asia, Central America, and Africa (CDC).
Hepatitis A and E are normally contracted from eating contaminated food or drinking contaminated water. Hepatitis B, C, and D are contracted through contaminated blood. These forms of hepatitis can be either acute or chronic, although types B and C usually become chronic.
Although Hepatitis E often causes an acute and self-limiting infection (in that it usually goes away by itself and the patient recovers) with low mortality rates in the western world, it bears a high risk of developing chronic hepatitis in immunocompromised patients with substantial mortality rates. Organ transplant recipients who receive immunosuppressive medication to prevent rejection are thought to be the main population at risk for chronic hepatitis E. Furthermore, in healthy individuals during the duration of the infection (usually several weeks), the disease severely impairs a person’s ability to work, care for family members, and obtain food. Hepatitis E occasionally develops into an acute, severe liver disease, and is fatal in about 2% of all cases. Clinically, it is comparable to hepatitis A, but in pregnant women the disease is more often severe and is associated with a clinical syndrome called fulminant hepatic failure. Pregnant women, especially those in the third trimester, suffer an elevated mortality rate from the disease of around 20%.
Hepatitis E is a viral hepatitis (liver inflammation) caused by infection with a virus called hepatitis E virus (HEV). HEV is a positive-sense single-stranded RNA icosahedral virus with a 7.5 kilobase genome. HEV has a fecal-oral transmission route. It is one of five known hepatitis viruses: A, B, C, D, and E. Infection with this virus was first documented in 1955 during an outbreak in New Delhi, India.A preventative vaccine (HEV 239) is approved for use in China.
History
The hepatitis E virus causes around 20 million infections a year. These result in around three million acute illnesses and as of 2010, 57,000 deaths annually. It is particularly dangerous for pregnant women, who can develop an acute form of the disease that is lethal in 20 percent of cases. The virus (HEV) is a major cause of illness and of death in the developing world and disproportionate cause of deaths among pregnant women.
The most recent common ancestor of Hepatitis E evolved between 536 and 1344 years ago. It diverged into two clades—an anthropotropic and an enzootic form—which subsequently evolved into genotypes 1 and 2 and genotypes 3 and 4 respectively. The divergence dates for the various genotypes are as follows: Genotypes 1/2 367–656 years ago; Genotypes 3/4 417–679 years ago. For the most recent common ancestor of the various viruses themselves: Genotype 1 between 87 and 199 years ago; Genotype 3 between 265 and 342 years ago; and Genotype 4 between 131 and 266 years ago. The anthropotropic strains (genotype 1 and 2) have evolved more recently than the others suggesting that this virus was originally a zooenosis.
The use of an avian strain confirmed the proposed topology of the genotypes 1–4 and suggested that the genus may have evolved 1.36 million years ago (range 0.23 million years ago to 2.6 million years ago). The use of a rat sequence also confirmed this topology and estimated date of divergence from the swine/human strains was 7.44×104 years ago (range 2.1×104 to 1.4×105 years ago). Since this date is approximately coincident with the advent of agriculture it may be that this virus originally infected rats and subsequently spread to pigs and then to humans. Additional work is required to support or refute this possibility as very few sequences have been isolated from species other than humans and suids.
Genotypes 1, 3 and 4 all increased their effective population sizes in the 20th century. The population size of genotype 1 increased noticeably in the last 30–35 years. Genotypes 3 and 4 population sizes began to increase in the late 19th century up to 1940–1945. Genotype 3 underwent a subsequent increase in population size until the 1960s. Since 1990 both genotypes' population sizes have been reduced back to levels last seen in the 19th century.
The overall mutation rate for the genome has been estimated at ~1.4×10−3 substitutions/site/year.
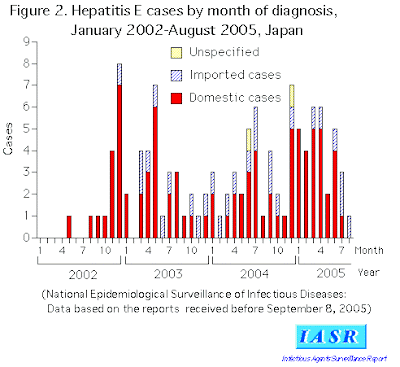
In 2004, there were two major outbreaks, both of them in sub-Saharan Africa. There was an outbreak in Chad in which, as of September 27, there were 1,442 reported cases and 46 deaths. The second was in Sudan with, as of September 28, 6,861 cases and 87 deaths. Increasingly, hepatitis E is being seen in developed nations, with reports of cases in the UK, US and Japan. The disease is thought to be a zoonosis in that animals are thought to be the source. Both deer and swine have been implicated.
In October 2007, an epidemic of hepatitis E was suspected in Kitgum District of northern Uganda where no previous epidemics had been documented. This outbreak has progressed to become one of the largest hepatitis E outbreaks in the world. By June 2009, the epidemic had caused illness in >10,196 persons and 160 deaths.
In 2011, a minor outbreak was reported in Tangail, a neighborhood of Dhaka, Bangladesh.
In June 2012, an outbreak was reported in city of Ichalkaranji, Maharashtra, India. As of June 14, 2012, 3232 cases were reported and 18 died. and 3 died in Shirol taluka of Kolhapur Maharashtra, India in June 2012. Officials in the Indian state of Maharashtra India suspect that contaminated water from the Panchganga river was responsible for the hepatitis E outbreak in Ichalkaranji.
In July 2012, an outbreak was reported in South Sudanese refugee camps in Maban County near the Sudan border. South Sudan's Ministry of Health reported over 400 cases and 16 fatalities as of September 13, 2012.Progressing further, as of February 2, 2013, 88 have died due to the outbreak. The "Medical charity Medecins Sans Frontieres (MSF) said it had treated almost 4,000 patients."
In April 2014 there was an outbreak in Nepal, Biratnagar Municipality with over 6,000 locals infected and at least 9 dead.

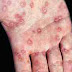
Signs and symptoms
Viral RNA becomes detectable in stool and blood serum during incubation period. Serum IgM and IgG antibodies against HEV appear just before onset of clinical symptoms. Recovery leads to virus clearance from the blood, while the virus may persist in stool for much longer. Recovery is also marked by disappearance of IgM antibodies and increase of levels of IgG antibodies.
 |
| Hepatitis E virus (HEV) particles in the cell culture supernatant of pork liver sausage sample A, collected at 33 dpi. A) Transmission electron micrograph |
While usually an acute disease, in immunocompromised
subjects—particularly in solid organ transplanted patients—hepatitis E
may cause a chronic infection. Occasionally this may cause liver fibrosis and cirrhosis.
Transmission
Hepatitis E is prevalent in most developing countries, and common in any country with a hot climate. It is widespread in Southeast Asia, northern and central Africa, India, and Central America. It is spread mainly by the fecal-oral route due to fecal contamination of water supplies or food; person-to-person transmission is uncommon.The incubation period following exposure to the hepatitis E virus ranges from three to eight weeks, with a mean of 40 days. Outbreaks of epidemic hepatitis E most commonly occur after heavy rainfalls and monsoons because of their disruption of water supplies. Major outbreaks have occurred in New Delhi, India (30,000 cases in 1955–1956), Burma (20,000 cases in 1976–1977), Kashmir, India (52,000 cases in 1978), Kanpur, India (79,000 cases in 1991), and China (100,000 cases between 1986 and 1988).
DEFRA said that there was evidence that the increase in hepatitis E in the UK was due to food-borne zoonoses, citing a study that found 10% of pork sausages on sale in the UK contained the virus. Some research suggests that food must reach a temperature of 70°C for 20 minutes to eliminate the risk of infection. An investigation by the Animal Health and Veterinary Laboratories Agency found hepatitis E in 49% of pigs in Scotland.
A number of other small mammals have been identified as potential reservoirs: the lesser bandicoot rat (Bandicota bengalensis), the black rat (Rattus rattus brunneusculus) and the Asian house shrew (Suncus murinus). A new virus designated rat hepatitis E virus has been isolated.
A rabbit hepatitis E virus has also been described, with a study published in 2014 showing that research rabbits from two different American vendors showed seroprevalences of 40% for Supplier A and 50% for Supplier B when testing for antibodies against hepatitis E virus (HEV). Supplier A was a conventional rabbit farm, and supplier B was a commercial vendor of specific pathogen free (SPF) research rabbits. The study remarks "HEV probably is widespread in research rabbits, but effects on research remain unknown." Laboratory animal care personnel, researchers, and support staff represent a new population at risk for HEV infection, and research facilities should be diligent in measures to prevention of this possibly zoonotic pathogen.
An avian virus has been described that is associated with hepatitis-splenomegaly syndrome in chickens. This virus is genetically and antigenically related to mammalian HEV, and probably represents a new genus in the family.
Treatment
Apart from supportive care, no specific validated treatment exists for
acute hepatis E infection. Although ribavarin is not registered for
Hepatitis E treatment, there is off-label experience for treating
chronic Hepatitis E with this compound. The use of low doses, 600 to 800
milligrams per day, of ribavirin over a three-month period has been associated with viral clearance in about two-thirds of chronic cases. Other possible treatments include peginterferon
or a combination of ribavirin and peginterferon. In general chronic HEV
infection is associated with immunosuppressive therapies, but
remarkably little is known about how different immunosuppressants affect
HEV infection. In one thirds of patients with solid-organ
transplantation viral clearance can be achieved by temporal reduction of
the level of immunosuppression. Calcineurin inhibitors (like cyclosporin) stimulate and mycophenolic acid
inhibit replication of Hepatitis E Virus and this should be considered
when physicians select immunosuppressive therapies for patients at risk
for Hepatitis E, for instance recipients of organ transplants.
Prevention
Improving sanitation is the most important measure in prevention of hepatitis E; this consists of proper treatment and disposal of human waste, higher standards for public water supplies, improved personal hygiene procedures and sanitary food preparation. Thus, prevention strategies of this disease are similar to those of many others that plague developing nations, and they require large-scale international financing of water supply and water treatment projects.A vaccine based on recombinant viral proteins was developed in the 1990s and tested in a high-risk population (military personnel of Nepal) in 2001. The vaccine appeared to be effective and safe, but development was stopped for economic reasons, since hepatitis E is rare in developed countries. There is no licensed hepatitis E vaccine for use in the US.
Although other HEV vaccine trials, including trials conducted in populations in southern Asia, have shown candidate vaccines to be effective and well-tolerated, these vaccines have not yet been produced or made available to susceptible populations. The exception is China. After more than a year of scrutiny and inspection by China's State Food and Drug Administration (SFDA), a hepatitis E vaccine developed by Chinese scientists was available at the end of 2012. This vaccine—called HEV 239 and sold as Hecolin by its developer Xiamen Innovax Biotech—was approved for prevention of hepatitis E in 2012 by the Chinese Ministry of Science and Technology, following a phase 3 trial on two groups of 50,000 people each from Jiangsu Province where none of the vaccinated became infected during a 12-month period, compared to 15 in the group given placebo treatment. The first vaccine batches came out of Innovax' factory in late October 2012, and will be sold to Chinese distributors.
References
- Zhou X, de Man RA, de Knegt RJ, Metselaar HJ, Peppelenbosch MP, Pan Q.; De Man; De Knegt; Metselaar; Peppelenbosch; Pan (2013). "Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review". Rev Med Virol. 23 (5): 295–304. doi:10.1002/rmv.1751. PMID 23813631.
- WHO. "Global Alert and Response (GAR); Hepatitis E". Retrieved 26 January 2012.
- Hoofnagle, J. H.; Nelson, K. E.; Purcell, R. H. (2012). "Hepatitis E". New England Journal of Medicine 367 (13): 1237–1244. doi:10.1056/NEJMra1204512. PMID 23013075.
- Bonnet, D.; Kamar, N.; Izopet, J.; Alric, L. (2012). "L'hépatite virale E : Une maladie émergente". La Revue de Médecine Interne 33 (6): 328–334. doi:10.1016/j.revmed.2012.01.017. PMID 22405325.
- Lu L, Li C, Hagedorn CH; Li; Hagedorn (2006). "Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis". Rev Med Virol 16 (1): 5–36. doi:10.1002/rmv.482. PMID 16175650.
- Miyahara K, Miyake Y, Yasunaka T et al. (2010). "Acute hepatitis due to hepatitis E virus genotype 1 as an imported infectious disease in Japan". Intern. Med. 49 (23): 2613–6. doi:10.2169/internalmedicine.49.4221. PMID 21139302.
- Doward, Jamie (21 September 2013). "Chefs fight for the right to serve their pork pink". The Observer newspaper. Retrieved 22 September 2013.
- "Hepatitis E Fact sheet".
- Satou K, Nishiura H; Nishiura (2007). "Transmission Dynamics of Hepatitis E Among Swine: Potential Impact upon Human Infection". BMC Vet. Res. 3: 9. doi:10.1186/1746-6148-3-9. PMC 1885244. PMID 17493260.
- Li TC, Chijiwa K, Sera N et al. (2005). "Hepatitis E Virus Transmission from Wild Boar Meat". Emerging Infect. Dis. 11 (12): 1958–60. doi:10.3201/eid1112.051041. PMC 3367655. PMID 16485490.
- Kuniholm MH & Nelson KE (2008). "Of Organ Meats and Hepatitis E Virus: One Part of a Larger Puzzle Is Solved". J Infect Dis 198 (12): 1727–1728. doi:10.1086/593212. PMID 18983247.
- "Wild Rats and Disease".
- Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A; Plenge-Bönig; Hess; Ulrich; Reetz; Schielke (March 2010). "Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR". J. Gen. Virol. 91 (Pt 3): 750–8. doi:10.1099/vir.0.016584-0. PMID 19889929.
- Cheng X, Wang S, Dai X, Shi C, Wen Y, Zhu M, Zhan S, Meng J; Wang; Dai; Shi; Wen; Zhu; Zhan; Meng (2012). "Rabbit as a novel animal model for hepatitis e virus infection and vaccine evaluation". PLoS ONE 7 (12): e51616. doi:10.1371/journal.pone.0051616. PMC 3521758. PMID 23272124





































0 comments:
Post a Comment