Hepatitis D
hepatitis D virus (HDV) and classified as Hepatitis delta virus, is a disease caused by a small circular enveloped RNA virus. It is one of five known hepatitis viruses: A, B, C, D, and E. HDV is considered to be a subviral satellite because it can propagate only in the presence of the hepatitis B virus (HBV). Transmission of HDV can occur either via simultaneous infection with HBV (coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state (superinfection).
Hepatitis D, also known as the delta virus, is an infection that causes your liver to swell. It is caused by the hepatitis D virus (HDV) and is uncommon in the United States.
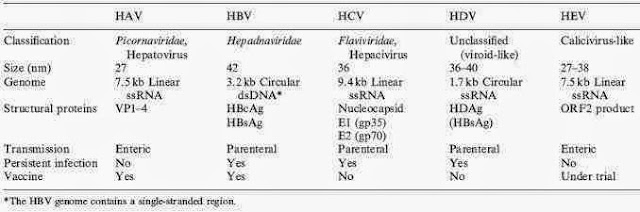
Hepatitis D is one of many forms of hepatitis—other types include
hepatitis A, B, C, and E. Unlike the other forms, hepatitis D cannot be
contracted on its own. You can only contract hepatitis D if you are
already infected with hepatitis B.
There is no cure for hepatitis D and no vaccine to prevent it.
Hepatitis D is one of many forms of hepatitis—other types include hepatitis A, B, C, and E. Unlike the other forms, hepatitis D cannot be contracted on its own. You can only contract hepatitis D if you are already infected with hepatitis B.
There is no cure for hepatitis D and no vaccine to prevent it.
Both
superinfection and coinfection with HDV results in more severe
complications compared to infection with HBV alone. These complications
include a greater likelihood of experiencing liver failure in acute
infections and a rapid progression to liver cirrhosis, with an increased chance of developing liver cancer in chronic infections In combination with hepatitis B virus, hepatitis D has the highest mortality rate of all the hepatitis infections, at 20%.
History
D virus was first reported in the mid-1977 as a nuclear antigen in patients infected with HBV who had severe liver disease. This nuclear antigen was then thought to be a hepatitis B antigen and was called the delta antigen. Subsequent experiments in chimpanzees showed that the hepatitis delta antigen (HDAg) was a structural part of a pathogen that required HBV infection to replicate. The entire genome was cloned and sequenced in 1986. It was subsequently placed in its own genus: Deltavirus.
Three genotypes (I–III) were originally described. Genotype I has been isolated in Europe, North America, Africa and some Asia. Genotype II has been found in Japan, Taiwan, and Yakutia (Russia). Genotype III has been found exclusively in South America (Peru, Colombia, and Venezuela). Some genomes from Taiwan and the Okinawa islands have been difficult to type but have been placed in genotype 2. However it is now known that there are at least 8 genotypes of this virus (HDV-1 to HDV-8). Phylogenetic studies suggest an African origin for this pathogen.
An analysis of 36 strains of genotype 3 estimated that the most recent common ancestor of these strains originated around 1930. This genotype spread exponentially from early 1950s to the 1970s in South America. The substitution rate was estimated to be 1.07×10−3 substitutions per site per year.
Genotype 8 has also been isolated from South America. This genotype is usually only found in Africa and may have been imported into South America during the slave trade.
Genotypes, with the exception of type 1, appear to be restricted to certain geographical areas: HDV-2 (previously HDV-IIa) is found in Japan, Taiwan and Yakoutia, Russia; HDV-4 (previously HDV-IIb) in Japan and Taiwan; HDV-3 in the Amazonian region; HDV-5, HDV-6, HDV-7 and HDV-8 in Africa
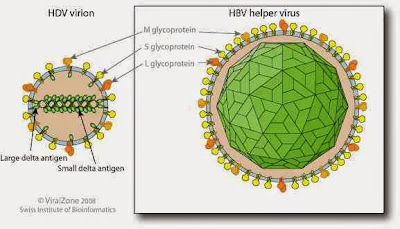
Structure of virus
A significant difference between viroids and HDV is that, while viroids
produce no proteins, HDV is known to produce one protein, namely HDAg.
It comes in two forms; a 27kDa large-HDAg, and a small-HDAg of 24kDa.
The N-terminals of the two forms are identical, they differ by 19 more
amino acids in the C-terminal of the large HDAg.
Both isoforms are produced from the same reading frame which contains
an UAG stop codon at codon 196, which normally produces only the
small-HDAg. However, editing by cellular enzyme adenosine deaminase-1
changes the stop codon to UCG, allowing the large-HDAg to be produced.
Despite having 90% identical sequences, these two proteins play
diverging roles during the course of an infection. HDAg-S is produced in
the early stages of an infection and enters the nucleus and supports
viral replication. HDAg-L, in contrast, is produced during the later
stages of an infection, acts as an inhibitor of viral replication, and
is required for assembly of viral particles.
Thus RNA editing by the cellular enzymes is critical to the virus’ life
cycle because it regulates the balance between viral replication and
virion assembly.
Signs and symptoms
The symptoms of hepatitis B and hepatitis D are similar, so it can
be difficult to tell which disease is causing your symptoms. Common
symptoms include:
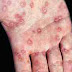
- yellowing of skin and eyes (jaundice)
- joint pain
- abdominal pains
- vomiting
- loss of appetite
- dark urine
- fatigue
Hepatitis D can also cause the symptoms of hepatitis B to worsen, or
appear in those who have been infected but haven’t yet developed
symptoms.
The virus can be acute (short-term) or chronic
(long-term). If you have chronic hepatitis D, you are at a higher risk
of developing complications from the disease. Long-term or chronic
hepatitis D might be present in your body for some time before the
symptoms develop.
If you have the chronic form of hepatitis B, you are more likely to develop chronic hepatitis D.
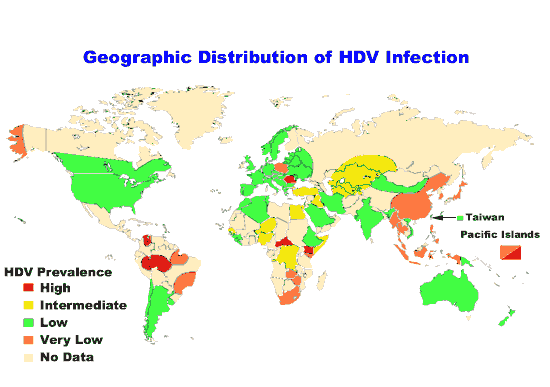
Transmission
transmission of hepatitis D are similar to those for hepatitis B.
Infection is largely restricted to persons at high risk of hepatitis B
infection, particularly injecting drug users and persons receiving
clotting factor concentrates. Worldwide more than 15 million people are
co-infected. HDV is rare in most developed countries, and is mostly associated with intravenous drug use.
However, HDV is much more common in the immediate Mediterranean region,
sub-Saharan Africa, the Middle East, and the northern part of South
America. In all, about 20 million people may be infected with HDV.
- yellowing of skin and eyes (jaundice)
- joint pain
- abdominal pains
- vomiting
- loss of appetite
- dark urine
- fatigue
The virus can be acute (short-term) or chronic (long-term). If you have chronic hepatitis D, you are at a higher risk of developing complications from the disease. Long-term or chronic hepatitis D might be present in your body for some time before the symptoms develop.
If you have the chronic form of hepatitis B, you are more likely to develop chronic hepatitis D.
Transmission
transmission of hepatitis D are similar to those for hepatitis B.
Infection is largely restricted to persons at high risk of hepatitis B
infection, particularly injecting drug users and persons receiving
clotting factor concentrates. Worldwide more than 15 million people are
co-infected. HDV is rare in most developed countries, and is mostly associated with intravenous drug use.
However, HDV is much more common in the immediate Mediterranean region,
sub-Saharan Africa, the Middle East, and the northern part of South
America. In all, about 20 million people may be infected with HDV.
Tests Used to Diagnose Hepatitis D
If you have symptoms of hepatitis D or B, you should see your doctor
for a diagnosis. If you have symptoms of the disease without jaundice,
your doctor may not suspect hepatitis.
Tell your doctor if you have been around anyone infected with
hepatitis or if you’ve traveled to a country where hepatitis B is
prevalent. According to the National Institutes of Health (NIH), you
are at particularly high risk if you (NIH):
- use IV drugs
- are a man who has sex with other men
- receive many blood transfusions
Your doctor will do a blood test to detect anti-hepatitis D antibodies in your blood. If antibodies are found, it means you have had exposure to the virus.
If your doctor suspects you have liver damage, he or she might also give you a liver function test.
This is a blood test that measures the levels of liver enzymes in your
blood. Results from this test will show if your liver is stressed or damaged.
Tell your doctor if you have been around anyone infected with hepatitis or if you’ve traveled to a country where hepatitis B is prevalent. According to the National Institutes of Health (NIH), you are at particularly high risk if you (NIH):
- use IV drugs
- are a man who has sex with other men
- receive many blood transfusions
If your doctor suspects you have liver damage, he or she might also give you a liver function test. This is a blood test that measures the levels of liver enzymes in your blood. Results from this test will show if your liver is stressed or damaged.
Complications of Hepatitis D
Complications of hepatitis D include:
- scarring of the liver (cirrhosis)
- liver disease
- liver cancer
Treatment and prevention
Low quality evidence suggests that interferon alpha can be effective in reducing the severity of the infection and the effect of the disease during the time the drug is given, but the benefit generally stops when the drug is discontinued, indicating that it does not cure the disease. Interferon is effective only in ~20% of cases.
The only known way to
prevent hepatitis D is to avoid infection with hepatitis B. There is a
vaccine for hepatitis B—which all children should receive. Adults who
are at high risk for infection, especially IV drug users, should also
be vaccinated.
Sources:
- Delta Agent (Hepatitis D). (2010). National Institutes of Health. Retrieved June 26, 2012, from http://www.meddean.luc.edu/lumen/MedEd/orfpath/virhepd.htm
- Hepatitis D. (2009). Centers for Disease Control and Prevention.Retrieved June 20, 2012, from http://www.cdc.gov/hepatitis/HDV/index.htm
- Hepatitis D. (n.d.). The Children’s Hospital of Philadelphia.Retrieved June 20, 2012, from http://www.chop.edu/service/viral-hepatitis-clinical-care-program/hepatitis-d.html#diagnosis
- Hepatitis D. (n.d.). World Health Organization.Retrieved June 20, 2012, from http://www.who.int/csr/disease/hepatitis/whocdscsrncs20011/en/index5.html
- Viral Hepatitis D. (n.d.). Loyola University Chicago.Retrieved June 20, 2012, from http://www.meddean.luc.edu/lumen/MedEd/orfpath/virhepd.htm
- Wang,
KS; Choo, QL, Weiner, AJ, Ou, JH, Najarian, RC, Thayer, RM, Mullenbach,
GT, Denniston, KJ, Gerin, JL, Houghton, M (1986 Oct 9–15). "Structure,
sequence and expression of the hepatitis delta (delta) viral genome". Nature 323 (6088): 508–14. doi:10.1038/323508a0. PMID 3762705.
- Fauquet, CM; Mayo MA; Maniloff J; Desselberger U; Ball LA (2005). "Deltavirus". Eight Report of the International Committee on Taxonomy of Viruses. London: 735–8.
- Poisson
F, Roingeard P, Baillou A, Dubois F, Bonelli F, Calogero RA, Goudeau A;
Roingeard; Baillou; Dubois; Bonelli; Calogero; Goudeau (November 1993).
"Characterization of RNA-binding domains of hepatitis delta antigen". J. Gen. Virol. 74 (Pt 11): 2473–8. doi:10.1099/0022-1317-74-11-2473. PMID 8245865.
- Zuccola
HJ, Rozzelle JE, Lemon SM, Erickson BW, Hogle JM; Rozzelle; Lemon;
Erickson; Hogle (July 1998). "Structural basis of the oligomerization of
hepatitis delta antigen". Structure 6 (7): 821–30. doi:10.1016/S0969-2126(98)00084-7. PMID 9687364.
- Saldanha JA, Thomas HC, Monjardino JP; Thomas; Monjardino (July 1990). "Cloning and sequencing of RNA of hepatitis delta virus isolated from human serum". J. Gen. Virol. 71 (7): 1603–6. doi:10.1099/0022-1317-71-7-1603. PMID 2374010.
- Elena SF, Dopazo J, Flores R, Diener TO, Moya A; Dopazo; Flores; Diener; Moya (July 1991). "Phylogeny of viroids, viroidlike satellite RNAs, and the viroidlike domain of hepatitis delta virus RNA". Proc. Natl. Acad. Sci. U.S.A. 88 (13): 5631–4. doi:10.1073/pnas.88.13.5631. PMC 51931. PMID 1712103.
- Sureau, C (2006). "The role of the HBV envelope proteins in the HDV replication cycle". Current topics in microbiology and immunology. Current Topics in Microbiology and Immunology 307: 113–31. doi:10.1007/3-540-29802-9_6. ISBN 978-3-540-29801-4. PMID 16903223.
- Yan
H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H,
Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W;
Zhong; Xu; He; Jing; Gao; Huang; Qi; Peng; Wang; Fu; Song; Chen; Gao;
Ren; Sun; Cai; Feng; Sui; Li (2012). "Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus". ELife 1: e00049. doi:10.7554/eLife.00049. PMC 3485615. PMID 23150796.
- Engelke,
M; Mills, K; Seitz, S; Simon, P; Gripon, P; Schnölzer, M; Urban, S
(April 2006). "Characterization of a hepatitis B and hepatitis delta
virus receptor binding site". Hepatology (Baltimore, Md.) 43 (4): 750–60. doi:10.1002/hep.21112. PMID 16557545.
- Schulze, A; Schieck, A; Ni, Y; Mier, W; Urban, S (February 2010). "Fine Mapping of Pre-S Sequence Requirements for Hepatitis B Virus Large Envelope Protein-Mediated Receptor Interaction". Journal of Virology 84 (4): 1989–2000. doi:10.1128/JVI.01902-09. PMC 2812397. PMID 20007265.
- Xia, YP; Yeh, CT, Ou, JH, Lai, MM (February 1992). "Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex". Journal of Virology 66 (2): 914–21. PMC 240792. PMID 1731113.
- Lehmann
E, Brueckner F, Cramer P; Brueckner; Cramer (November 2007). "Molecular
basis of RNA-dependent RNA polymerase II activity". Nature 450 (7168): 445–9. doi:10.1038/nature06290. PMID 18004386.
- Filipovska J, Konarska MM; Konarska (January 2000). "Specific HDV RNA-templated transcription by pol II in vitro". RNA 6 (1): 41–54. doi:10.1017/S1355838200991167. PMC 1369892. PMID 10668797.
- Greco-Stewart,
VS; Schissel, E; Pelchat, M (2009-03-30). "The hepatitis delta virus
RNA genome interacts with the human RNA polymerases I and III". Virology 386 (1): 12–5. doi:10.1016/j.virol.2009.02.007. PMID 19246067.
- Delta Agent (Hepatitis D). (2010). National Institutes of Health. Retrieved June 26, 2012, from http://www.meddean.luc.edu/lumen/MedEd/orfpath/virhepd.htm
- Hepatitis D. (2009). Centers for Disease Control and Prevention.Retrieved June 20, 2012, from http://www.cdc.gov/hepatitis/HDV/index.htm
- Hepatitis D. (n.d.). The Children’s Hospital of Philadelphia.Retrieved June 20, 2012, from http://www.chop.edu/service/viral-hepatitis-clinical-care-program/hepatitis-d.html#diagnosis
- Hepatitis D. (n.d.). World Health Organization.Retrieved June 20, 2012, from http://www.who.int/csr/disease/hepatitis/whocdscsrncs20011/en/index5.html
- Viral Hepatitis D. (n.d.). Loyola University Chicago.Retrieved June 20, 2012, from http://www.meddean.luc.edu/lumen/MedEd/orfpath/virhepd.htm
- Wang,
KS; Choo, QL, Weiner, AJ, Ou, JH, Najarian, RC, Thayer, RM, Mullenbach,
GT, Denniston, KJ, Gerin, JL, Houghton, M (1986 Oct 9–15). "Structure,
sequence and expression of the hepatitis delta (delta) viral genome". Nature 323 (6088): 508–14. doi:10.1038/323508a0. PMID 3762705.
- Fauquet, CM; Mayo MA; Maniloff J; Desselberger U; Ball LA (2005). "Deltavirus". Eight Report of the International Committee on Taxonomy of Viruses. London: 735–8.
- Poisson F, Roingeard P, Baillou A, Dubois F, Bonelli F, Calogero RA, Goudeau A; Roingeard; Baillou; Dubois; Bonelli; Calogero; Goudeau (November 1993). "Characterization of RNA-binding domains of hepatitis delta antigen". J. Gen. Virol. 74 (Pt 11): 2473–8. doi:10.1099/0022-1317-74-11-2473. PMID 8245865.
- Zuccola HJ, Rozzelle JE, Lemon SM, Erickson BW, Hogle JM; Rozzelle; Lemon; Erickson; Hogle (July 1998). "Structural basis of the oligomerization of hepatitis delta antigen". Structure 6 (7): 821–30. doi:10.1016/S0969-2126(98)00084-7. PMID 9687364.
- Saldanha JA, Thomas HC, Monjardino JP; Thomas; Monjardino (July 1990). "Cloning and sequencing of RNA of hepatitis delta virus isolated from human serum". J. Gen. Virol. 71 (7): 1603–6. doi:10.1099/0022-1317-71-7-1603. PMID 2374010.
- Elena SF, Dopazo J, Flores R, Diener TO, Moya A; Dopazo; Flores; Diener; Moya (July 1991). "Phylogeny of viroids, viroidlike satellite RNAs, and the viroidlike domain of hepatitis delta virus RNA". Proc. Natl. Acad. Sci. U.S.A. 88 (13): 5631–4. doi:10.1073/pnas.88.13.5631. PMC 51931. PMID 1712103.
- Sureau, C (2006). "The role of the HBV envelope proteins in the HDV replication cycle". Current topics in microbiology and immunology. Current Topics in Microbiology and Immunology 307: 113–31. doi:10.1007/3-540-29802-9_6. ISBN 978-3-540-29801-4. PMID 16903223.
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W; Zhong; Xu; He; Jing; Gao; Huang; Qi; Peng; Wang; Fu; Song; Chen; Gao; Ren; Sun; Cai; Feng; Sui; Li (2012). "Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus". ELife 1: e00049. doi:10.7554/eLife.00049. PMC 3485615. PMID 23150796.
- Engelke, M; Mills, K; Seitz, S; Simon, P; Gripon, P; Schnölzer, M; Urban, S (April 2006). "Characterization of a hepatitis B and hepatitis delta virus receptor binding site". Hepatology (Baltimore, Md.) 43 (4): 750–60. doi:10.1002/hep.21112. PMID 16557545.
- Schulze, A; Schieck, A; Ni, Y; Mier, W; Urban, S (February 2010). "Fine Mapping of Pre-S Sequence Requirements for Hepatitis B Virus Large Envelope Protein-Mediated Receptor Interaction". Journal of Virology 84 (4): 1989–2000. doi:10.1128/JVI.01902-09. PMC 2812397. PMID 20007265.
- Xia, YP; Yeh, CT, Ou, JH, Lai, MM (February 1992). "Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex". Journal of Virology 66 (2): 914–21. PMC 240792. PMID 1731113.
- Lehmann E, Brueckner F, Cramer P; Brueckner; Cramer (November 2007). "Molecular basis of RNA-dependent RNA polymerase II activity". Nature 450 (7168): 445–9. doi:10.1038/nature06290. PMID 18004386.
- Filipovska J, Konarska MM; Konarska (January 2000). "Specific HDV RNA-templated transcription by pol II in vitro". RNA 6 (1): 41–54. doi:10.1017/S1355838200991167. PMC 1369892. PMID 10668797.
- Greco-Stewart, VS; Schissel, E; Pelchat, M (2009-03-30). "The hepatitis delta virus RNA genome interacts with the human RNA polymerases I and III". Virology 386 (1): 12–5. doi:10.1016/j.virol.2009.02.007. PMID 19246067.

































I was diagnosed as HEPATITIS B carrier in 2013 with fibrosis of the
ReplyDeleteliver already present. I started on antiviral medications which
reduced the viral load initially. After a couple of years the virus
became resistant. I started on HEPATITIS B Herbal treatment from
ULTIMATE LIFE CLINIC (www.ultimatelifeclinic.com) in March, 2020. Their
treatment totally reversed the virus. I did another blood test after
the 6 months long treatment and tested negative to the virus. Amazing
treatment! This treatment is a breakthrough for all HBV carriers.